Stomata are tiny little pores, predominantly found in the epidermis of the leaf. The word is borrowed from Greek and means mouth, which is rather suitable, as this is where the plant is “ inhaling” carbon dioxide and “exhaling” oxygen. The stoma (singular) opens to take in the carbon dioxide. When and how much it opens is determined by environmental factors and hormone signals.
how many stomata a plant has depend on a range of factors but one of them is the availability of carbon dioxide in the atmosphere. When the levels of carbon dioxide are high the plant doesn’t need so many stomata in order to process enough carbon dioxide. But when there is not so much carbon dioxide available the plant needs many stomata. The plant can regulate the amount of stoma in the new leaf growth.
Developing stomata is ”costly” for the plant, every stoma uses quite a lot of water, through transpiration. So if the access to water is limited the plant can’t afford to develop many stomata.
Margret Steintorsdottir’s research revolves around using paleonthological stoma samples to assess the carbon dioxide levels in the atmosphere over time, in order to get a better idea of how the climate has been changing over the last 270 million years. Margret’s research provides a very interesting opportunity to get a deeper understanding of how animals and plants collaborate and have collaborated throughout history.
Through familiarising myself with her research, more questions arose in my mind. What if there is another level to this collaborative process? Sure we are Entirely dependent on each other for breathing and in that sense plants are working with us to take care of the things that we need to remove from our system and use it to produce the things we need to put into our system. This goes for the chemical composition of the air, as well as the food-compost cycle. But what if the plants are more or less consciously working to keep the levels of Carbon dioxide in check? Helping us to maintain a climate that is habitable, not only for them selves but also for us, by increasing the amount of stomata to accommodate for quicker processing when required?
Would plants that are cared for and regularly watered have a higher tendency of helping us out with this conversion since they can “afford” to develop more stomata when they are being looked after? If so how would this affect the development of stomata amongst a certain, commonly cultivated, species over time?
To look further into this I choose to take samples from cultivated and not cultivated species of plants of same family. Species that have evolved alongside one and other in a similar climate and with similar conditions, with the only difference that one has been extensively used and cared for by humans whilst the other has remained outside of our cultural interest and thus left alone.
I collected samples from the same plant during a range of conditions, in order to ensure I have a complete data set. I collected before and after rain, in the daytime and nighttime, from the over and underside of the leaf and from a new leaf as well as an older leaf. All to get a better idea of how the stomata are distributed in the plant and how they are used by the plant. And of course, primarily, how they differ (and what similarities I can observe) between species.
It would of course be very interesting to look at samples from the same plant over a very long period of time, whilst monitoring the climate and conditions that the plant is experiencing. But that’s for another time.
For the purpose of this project I am collecting samples from a fairly narrow selection of species. Some commonly cultivated and others close relatives of commonly cultivated species. I chose species that have been used by humans for a range of purposes, not just agriculture, in an attempt to get data from plants that really have been cared for, as I imagine herbs and medicinal plants would be, and not exploited to the extent that some of our most common cash crops are. There is indeed another interesting comparison to make, but once again for another time.

When collecting samples (or harvesting anything from a plant) I try to make a point of asking for permission and explaining why I wish to take something from them. Some would say that plants can’t answer back but to me it makes sense and I think it’s good practice to communicate with non humans and to assume that they can understand even if they may not respond with words. It helps me to remain sensitive and open enough to experience the surrounding nature vividly and with a high sense of connectedness.
In order to be able to travel with my samples, as well as store them for an undefined period of time whilst working on this project, I opted to use a technique that I call micro moulds. I am using a material that would ordinarily be used by dental surgeons to make casts of teeth.

The quick drying paste is used to make a negative three dimensional impression of the leaf. Later on I can make a positive cast of the imprint and end up with an artificial replica of the sample, with high enough levels of detail for features on the scale of 1nm to be clearly visible. Pretty cool huh?!

I have now travelled across the sea to Melbourne, Australia. Here I am using a scanning electron microscope at the Royal Melbourne Institute of Technology, to go through my samples and assess the material I have collected during my time in the field.
when I made the first test samples in Stockholm, in 2018, I came across an epoxy resin for making the casts. It was supposed to be suitable for withstanding the harsh conditions that a scanning electron microscope imposes on the samples. Here at RMIT they do not have the same resin but I have been advised to try nail polish as it should theoretically provide a similar result.
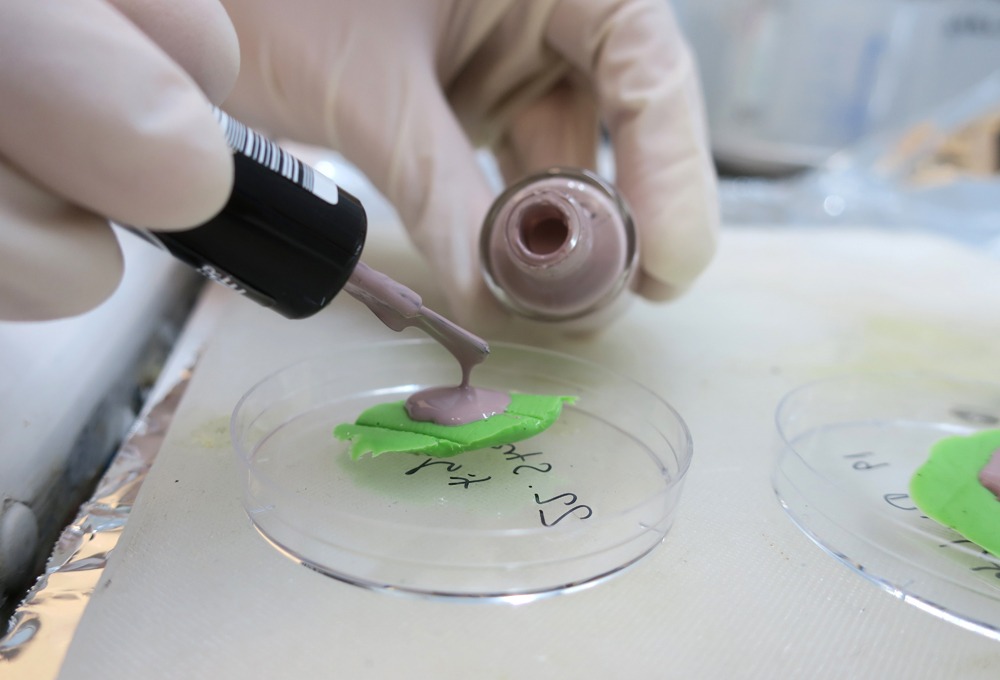

The samples were coated with a thin layer of iridium before I loaded them into the microscope.

But it proved to be a lot more complicated than I had hoped. The Nail Polish works fine in terms of preserving detail in the casts but it melts in the high voltage. Many many hours I have now spent trying to combat this issue from a range of angles, trying everything that I can think of. But with little success. Frustrated and disappointed I return to the drawing board. Which in this case is the kitchen table in the family home of Sam and Anna, who’s house we are minding whilst they are away on Christmas holidays. In fact everyone in the world seems to be away for fun filled celebrations with family and friends. I on the other hand am stuck in a temperature controlled and artificially lit laboratory, trying to make the most of being left alone with the equipment for a few weeks while the facilities are officially closed.

With the help from RMIT technician Peter Rummel I find a recipe for another kind of resin that might possibly work.
I mix up a batch, drop it onto my samples and let it try for a few days, then repeat the sample preparation and coating procedure.
Finally I get an image!

But the sample is still unstable and I can’t get the image quality that I want. The method is not optimal and I conclude that it is time for me to resign.
After Some encouraging conversations with my friend Pablo, another RMIT technician, I agree to just take a break. Which turns out to be timely anyway since me and Peter are due to travel up the east coast to build an art space for a music festival in the coming weeks.
So this is it for now. Peter, myself and the 21 selected samples are returning to Sweden in a few weeks time. Our arrival will mark the beginning of the rest of the project. More chapters will follow – on the research, the plants, ethnobotany and of course the continuation of the lab work. Keep checking in from time to time and keep in touch with me via social media. Perhaps you see something that I don’t. Maybe there is a research question you would like to explore. I am always interested to hear your thoughts, questions and comments.
Lots of love to you all for now.
Over and out